Éléktron
(Salinan ti vérsi basa Inggris)
- Pikeun kagunaan séjén, tempo Éléktron (disambiguasi).
| Electron | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
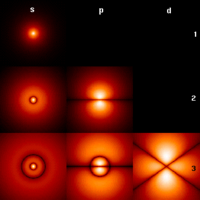 The first few hydrogen atom electron orbitals shown as cross-sections with color-coded probability density | ||||||||||
| Classification | ||||||||||
| ||||||||||
| Properties | ||||||||||
|
Éléktron (ogé disebut négatron, biasa dilambangkeun ku e−) mangrupa partikel subatomik. Dina atom, éléktron ngurilingan inti proton jeung neutron dina sarupaning konfigurasi éléktron.
Éléktron mangrupa salah sahiji golongan partikel subatomik nu disebut lepton nu dipercaya mangrupa partikel fundaméntal (nyaéta teu bisa dibeulah deui jadi bagéan nu leuwih leutik).
Éléktron mibanda spin 1/2, nu nunjukkeun yén éléktron téh hiji fermion, hartina, nuturkeun statistik Fermi-Dirac.
Dina mékanik kuantum, éléktron digambarkeun ku Dirac Equation. Dina Modél Baku fisika partikel, éléktron ngabentuk hiji doblét dina SU(2) jeung electron neutrino, as they interact through the weak interaction. The electron has two more massive partners, with the same charge but different masses: the muon and the tauon.
The antimatter counterpart of the electron is its antiparticle, the positron. The positron has the same amount of electrical charge as the electron, except that the charge is positive. It has the same mass and spin as the electron. When an electron and a positron meet, they may annihilate éach other, giving rise to two gamma-ray photons, éach having an energy of 0.511 MeV (511 keV). See also Electron-positron annihilation.
Some théorists believe the electron may be a very small black hole.
Sipat ganda
[édit | édit sumber]Éléktron bisa némbongkeun boh sipat partikel jeung gelombang. Éléktron nu kabeungkeut na inti polahna salaku standing wave.
Details
[édit | édit sumber]The electron has a negative electric charge of -1.6 × 10−19 coulombs, and a mass of about 9.10 × 10-31 kg (0.51 MeV/c2), which is 1/1800 of the proton mass.
It is believed that the number of electrons that would fit in the known universe is 10 followed by 130 zeros.
Listrik
[édit | édit sumber]When electrons move, free of the nuclei of atoms, and there is a net flow, this flow is called electricity, or an electric current. This might be compared to a flock of sheep moving north together, while the shepherds do not. Electric charge can be directly méasured with an electrometer. Electric current can be directly méasured with a galvanometer.
So-called "static electricity" is not a flow of electrons at all. More correctly called a "static charge", it refers to a body that has more or fewer electrons than are required to balance the positive charge of the nuclei. When there is an excess of electrons, the object is said to be "negatively charged". When there are fewer electrons than protons, the object is said to be "positively charged". When the number of electrons and the number of protons are equal, the object is said to be electrically "neutral".
Sajarah
[édit | édit sumber]The electron had been posited by G. Johnstone Stoney, as a unit of charge in electrochemistry, but Thompson réalised that it was also a subatomic particle.
The electron was discovered by J.J. Thomson in 1897 at the Cavendish Laboratory at Cambridge University, while studying "cathode rays." Influenced by the work of James Clerk Maxwell, and the discovery of the X-ray, he deduced that cathode rays existed and were negatively charged "particles", which he called "corpuscles".
Tempo ogé
[édit | édit sumber]Tumbu kaluar
[édit | édit sumber]- Particle Data Group
- Stoney, G. Johnstone, "Of the 'Electron,' or Atom of Electricity". Philosophical Magazine. Series 5, Volume 38, p. 418-420 October 1894.
